
A joint research team from the Institute of Biophysics of the Chinese Academy of Sciences and the Beijing Institute of Technology has uncovered a pivotal mechanism by which bacteria defend themselves against viral infection.
The study, published in Cell on May 8, reveals how cyclic dinucleotides (CDNs), synthesized during activation of the cyclic oligonucleotide-based anti-phage signaling system (CBASS) immune mechanism, trigger the filamentous assembly of phospholipase effectors, which execute the downstream immune response by disrupting membranes.
CBASS is a crucial innate antiviral defense mechanism in bacteria, with core effector modules that are evolutionarily conserved across both prokaryotic and eukaryotic immunity. While previous research had identified CDNs as key signaling molecules, the precise molecular details of how they activate downstream effectors remained unclear.
Focusing on CapE, a representative phospholipase effector in the CBASS system, the researchers employed an integrative approach combining cryo-electron microscopy and X-ray crystallography to determine its structure in three distinct states: an inactive dimer, a CDN-bound higher-order assembly, and a substrate-analog-bound catalytic mimic. Together, these structural snapshots capture the full conformational transition underlying CapE activation.
The findings show that upon binding to CDNs, CapE experiences a dramatic structural rearrangement. This transformation exposes its catalytic site and promotes its polymerization into ordered filaments. These filaments serve as active platforms for phospholipid cleavage, enabling rapid and robust activation of the bacterial immune response.
Further experiments involving structure-guided mutagenesis confirmed that both the formation of filaments and enzymatic activity are essential for CBASS-mediated membrane disruption and programmed cell death. These results underscore the functional significance of the activation mechanism.
By establishing a direct molecular link between CDN sensing and effector activation, the study offers a unified model for how CDNs trigger membrane-targeting immune responses. Moreover, it highlights filament formation as a broadly conserved strategy for regulating enzymatic activity across diverse immune systems.
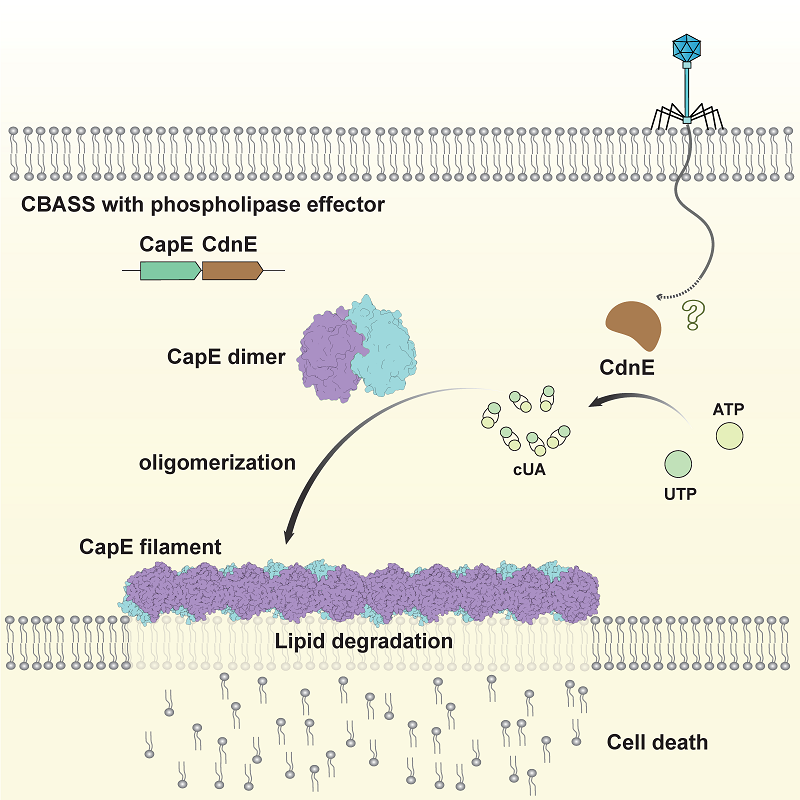
Model of anti-phage defense by CapE (Image by GAO Pu's group)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

